ICP-MS Laboratory
Contact
Tyrone Rooney
Department of Earth and Environmental Sciences
288 Farm Lane, Room 207
East Lansing, MI 48824
Phone: 517-432-5522
Email: rooneyt@msu.edu
The Department of Earth and Environmental Sciences at Michigan State University operates a Thermo Scientific ICAP Q quadrupole Inductively Coupled Plasma Mass Spectrometer (ICP-MS). The ICP-MS is principally used in combination with a Photon Machines Analyte G2 193 nm excimer laser ablation system equipped with a 15 x 15 cm HelEx sample cell, for solid sample micro-analyses. The apparatus is most frequently used for minerals, glasses, fused rock powders but may also be used a variety of other solid materials including certain metal alloys, ceramics, bones, or teeth. Liquid sample solutions also can be analyzed. Then, a CETAC ASX 520 autosampler is available and may hold >100 samples.
Our ICAP Q instrument is equipped with a collision cell, which minimizes interferences caused by argon molecules (e.g., 40Ar35Cl+ on 75As+, N2+ on 28Si+, 40Ar16O1H+ on 57Fe+) and substantially improves detection limits on interference-prone light mass isotopes such as first-row transition metals. The instrument has proven to be capable of analyzing Na, Mg, Al, Si, P, K, Ca, Fe and As with precision and accuracy comparable to those of the heavier atomic mass elements that are conventionally analyzed by ICP-MS (<±5%, or better for matrix-matched samples for which several calibration materials are available). Detection limits vary, generally in the ppb range for light masses and the ppt range for high masses in solution, and ppm to ppb ranges in laser (beam size dependent). Please note that these detection limits are only achievable if solutions are prepared in ultra-clean water and trace-metal grade solvents, and that instrumental precision is generally reduced when working at or near detection limit.
The ICP-MS is capable of analyzing most elements, either as single elements or in multi-element analysis, with high precision and sensitivity. The analyses can be semi-quantitative or quantitative.
The ICAP Q ICP-MS is located in the Food Safety and Toxicology building at MSU in a clean laboratory. The clean environment of the room complements the high sensitivity of the instrument.

Our instrumentation setup with ICP-MS, auto-sampler and laser (l to r)

The ICAP Q ICP-MS, see manufacturer’s webpage for more details.


The Photon-Machines Analyte G2 excimer laser and the HelEx sample holder, with multiple glass samples mounted. The use of a large cell and large sample holder reduces the need for sample holder exchange, while increasing the degree of automation. A sample holder with different design can hold several 1-inch mounts, 1 cm mounts, and petrographic thin sections. See manufacturer’s webpage for more details.
ICP-MS instruments have become popular in analytical laboratories owing to their versatility. In a few minutes, the ICP-MS can produce high quality data for elements with wide range of atomic masses, from 6Li to 238U. The best results are obtained for elements that have ionization potentials lower than those of the carrying gas (Ar, 15.8 eV) and that are free of isobaric interferences. The most common applications for ICP-MS are in biological, environmental, geological, and industrial fields. The following is a modest list of materials that can be analyzed by ICP-MS:
Archeological Applications: artifacts (e.g. ancient ceramics, metals and alloys) and raw materials (rocks, soil, bones, teeth)
Biological Applications: Animals and plant tissues and fluids (digested and dissolved), teeth, bones, etc…
Foods: beverages, food packaging, metabolites, flour, seafood, wine.
Health sciences: medicines, chemotherapy drugs, toxicology studies, dietary supplements, human tissues and fluids.
Earth Science Applications: fossils, minerals, meteorites, rocks, soil, water.
Environmental Applications: atmospheric deposits (wet, dry), brines, car exhaust particles, coal fly ash, dust, gases from landfills, incinerator wastes, organic waste, oil pollution, paint, snow, sludge, washing powders.
Forensic Science: glass, illicit drugs, soils, paint, metals.
Industrial Applications: alloys, automobile catalytic converters, ceramics, dyes, glass, lab gloves, nuclear industry products, paint, paper, petroleum based products, plastic, rare earth element compounds, steel, silica, superconductors, sulfides.
Our ICP-MS lab is primarily focused and optimized for geological applications, but can accommodate projects in multiple domains. Please contact us about applications in any other domains.
Our methods: principles of ICP-MS analysis
Samples are placed under helium flux in the HelEx sample chamber. A laser beam (here with a 193 nm wavelength) is focused on the sample surface and turns it into an aerosol. The sample chamber moves at pre-set locations and may move as the laser is firing, allowing ablations to be done either as a spot (drill) or as a surface scan. Our laser beam size can vary from 160 microns to 5 microns, and will be optimized based on sample size and analytical concentrations. A collection cup lies above the sample, where high-purity He gas entrains the ablated material and conveys it to the ICP-MS.
In the ICP-MS injection chamber, the He-carried sample is mixed with argon and conveyed inside a quartz torch. Additional argon is introduced in the torch around the sample injector. An induction coil generates an electro-magnetic field such that the argon-sample mixture is ionized as a plasma. The plasma is introduced into the mass spectrometer through a set of cones (sampling cone and skimmer cone). Ions of interest are filtered through a set of lenses and carried to the quadrupole for detection. On modern ICP-MS such the ICAP Q, prior to detection, ions circulate through a He-fluxed collision cell, where molecular compounds are disintegrated in order to reduce mass interferences with analyzed ions.
Solution mode ICP-MS is simpler as the laser ablation process is bypassed entirely. Instead, the solution is aspired and conveyed into a nebulizer by a peristaltic pump. There, it is mixed with argon and then introduced inside the torch for ionization.
Solution mode ICP-MS is simpler as the laser ablation process is bypassed entirely. Instead, the solution is aspired and conveyed into a nebulizer by a peristaltic pump. There, it is mixed with argon and then introduced inside the torch for ionization. Automation of the sample introduction process is made possible by the use of an autosampler.
Our methods: data generation principles
Whether the instrument is used in solution or laser mode, the following steps are required in order to generate exploitable data:
- For each investigated element, the ICP-MS measures the abundance of one of its isotopes (generally expressed in counts per second, or cps). To relate measured intensities to actual concentrations, calibration must be performed at the beginning of the analytical session. Calibration is done using multiple standards with a matrix similar to that of the samples. Standards can consist of solutions spiked with precisely known amounts of one, or several, trace elements, solutions of reference materials of precisely known trace element compositions (e.g., NIST SRM 1640). These standards can be diluted to extend calibration bounds and better reflect sample concentrations, if necessary. For laser ablation, natural reference rock materials (e.g., USGS BHVO-1G), artificial glass doped with precisely known amounts of trace elements (e.g., NIST SRM 612, USGS GSD-1G), are used in combination with natural reference rock powders fused in lithium tetraborate similar to samples analyzed for whole rock compositions, for a total of ~18 calibration standards (exact routine may vary). For each element, the standards with the highest concentrations are run as unknowns for extrapolation quality assessment purposes.
- For each standard and sample analyzed, a few elements of previously known concentrations must be analyzed to precisely estimate possibly variable sample introduction parameters such as plasma uptake rate by the mass spectrometer cones, solution uptake rate by the autosampler, or ablation rate by the laser. These internal standards consist of a multi-element spike solution (e.g., a Sc-Ge-Y-In-Tb-Bi solution) added to the sample and standard solutions, for solution ICP-MS. In laser ablation, internal standards normally consist of major elements of which concentrations in the sample have previously been determined, usually by X-ray fluorescence in our laboratories. Laser ablation internal standards may include Mg, Si, Ca, Ti, Fe. Alternatively, if no previous analyses exist to allow the use of the internal standard method, the sum of the concentration of all major elements can be used as internal standard, this is made possible by the capability of the ICAP Q to precisely and accurately measure all light mass isotopes in collision cell mode. Intensities of the analytes are normalized to the intensities of the internal standard elements. Using several internal standards better addresses uncertainties associated with variable isotopic masses, ionization and/or ablation characteristics of the analytes.
- In solution mode ICP-MS, to precisely assess quantification limits in the samples, a laboratory blank must be added within the analytical session. The blank must be chemically processed the exact same way as the samples so that possible contamination from the solvents or containers can be quantified and blank corrections applied to the samples.
- A pair of standards is periodically analyzed as unknowns throughout an analytical session, in order to monitor instrumental drift and, if necessary, correct the intensities obtained on the samples for instrumental drift. Instrumental drift means a change over time in the instrumental sensitivity to an analyte, or practically, a change in the ratio measured between an analyte and its internal standard.
-
The joint X-ray Fluorescence and ICP-MS Laboratories at MSU offer a variety of analytical services, including elemental packages for rock and soil samples (Option 1 below).
The following schedule shows estimated fees. Additional discounts may be given for large numbers of samples or in-house sample preparation. Fees are subject to change at any time. Prices reflect a normal turn-around time of about two (2) months. Rush orders may require additional charges. Please consult Dr. Tyrone Rooney before officially quoting any prices.
Geological (rock and soil) samples by X-ray fluorescence and laser-ablation ICP-MS
-
Sample Preparation: All samples are analyzed as glass disks, prepared by fusion of finely-ground rock powders with lithium tetraborate. Samples may be submitted as powders or in bulk. Submit a minimum of 8 grams powder or 30 grams whole rock.
Users may also request to prepare their own samples at MSU, subject to a $2.00/sample materials charge.
Major and trace element packages (per sample).
Elements
Academic or research
Commercial
Major elements only* (XRF)
$40
$80
Major elements + 30 trace elements** (XRF + LA-ICP-MS)
$100
$200
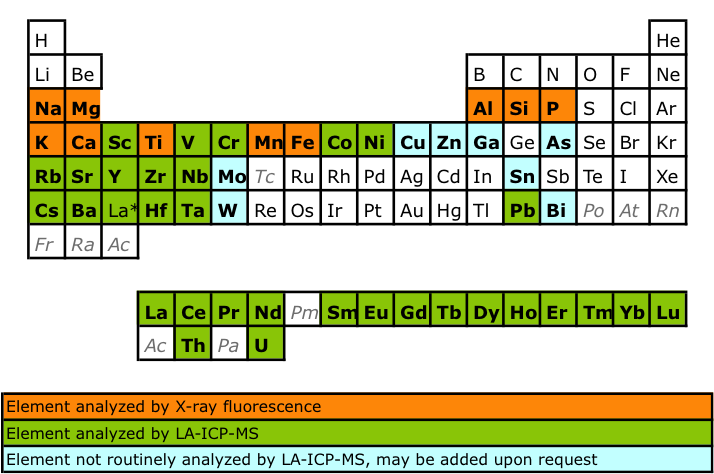
*Major element package includes SiO2, TiO2, Al2O3, Fe2O3, MgO, MnO, CaO, Na2O, K2O, P2O5.
**Trace element package includes Sc, V, Cr, Co, Ni, Rb, Sr, Y, Zr, Nb, Cs, Ba, all REE, Hf, Ta, Pb, Th, U. We target and normally exceed 5% precision (relative standard deviation) on trace element analyses, by analyzing 3 replicates of all samples, and a pair of standards processed as unknowns multiple times throughout the analytical session. Analyses of our standards as unknowns routinely return concentrations within < 5% of GEOREM compiled values. Additional trace elements can be included upon request, although precision and accuracy may vary. Contact us for inquiries.A minimum charge of $350 (academic) and $600 (commercial) will apply for all geological analyses involving LA-ICP-MS. This cost reflects the minimum time to tune the instrument, run standards, and data processing.
-
A minimum charge of $350 (academic) and $600 (commercial) will apply for all geological analyses involving LA-ICP-MS. This cost reflects the minimum time to tune the instrument, run standards, and data processing.
Please also factor in the following sample preparation costs, to be added to the costs listed above:
Sample preparation fees for any type of elemental analysis.
Sample type
Cost per sample
Trimming/powdering of bulk samples*
$5/sample
Li2B4O7 fusion (of powdered samples**)
$6.50/sample
*Trimming/powdering services are only offered if analysis by XRF and/or ICP-MS in our laboratories is desired. WE DO NOT OFFER TRIMMING/POWDERING OR FUSING SERVICES ALONE.
**Submitted sample powders should be fine enough so they are not “gritty” when rubbed between sheets of paper. Re-powdering of gritty samples will require an additional charge. Fusion will only be performed for samples to be subsequently analyzed in our laboratories.
ICP-MS (solution) Samples
Samples must be free of solids, and in a matrix of 1-2% HNO3. A minimum of 10 mL should be submitted for each sample. It is preferred that the user provides standard solutions spanning the range of concentrations of elements of interest and that the samples and standards are spiked with a precisely known amount of spike solution of elements to be used as internal standards (please contact us with questions, and read sample preparation guidelines below). If standards are not provided, samples are not spiked, and/or concentrations are not known, additional sample preparation charges may apply.
Please contact us for a quote. Charges will depend on the amount of sample preparation needed, analysis time and amount of data processing required.
LA-ICP-MS Samples
Laser ablation ICP-MS will be selected over solution ICP-MS for solid samples (e.g., fused powders, or in-situ analyses of minerals, glass, ceramics, etc.). Based on the relative complexity of analyses (number of ablations, standardization, data processing, etc.) rates will vary for solid sample introduction.
Please contact us for a quote. Charges will depend on the amount of sample preparation needed, analysis time and amount of data processing required. A minimum charge of $350 (academic) and $600 (commercial) will apply for all analyses involving LA-ICP-MS.
Sample Preparation and Standards
Laser Ablation Sample Preparation
In general, one of the largest benefits of laser ablation ICP-MS is that sample preparation is minimal, as long as the sample is relatively homogenous or the region of interest can be identified in the sample (e.g. minerals in thin section). It is much preferred that the concentration of at least one element is precisely known, such that it can be used as an internal standard. Please consult with lab manager for any special sample preparation required for specific types of samples, or specific standards that may be needed. Requests to analyze certain types of samples or matrices (notably certain metals, alloys and metal sulfides) may be declined in order to protect the instrumental set up long-term cleanliness and the ICP-MS high sensitivity to very low concentrations.
Solution Sample Preparation
Sample preparation is responsibility of the user. Always discuss the sample preparation procedure with the lab manager to make sure that the procedure is compatible with the ICP-MS. The following general rules should be followed:
- The solutions to be run in the ICP-MS should be 1% - 2% HNO3 if possible.
- Reagents used for the sample preparation should be double distilled or ultra pure. The high purity acids reduce contamination and background levels in the instrument.
- Total dissolved solids of solutions should be less than 0.1% (the total dissolved solids is the ratio of the sample weight to the solution volume).
- We CANNOT ACCEPT seawater samples, to prevent corrosion in the ICP-MS and ensure its longevity.
- Blank solution: The user should provide a procedural blank. This blank should be prepared by the same method used to prepare the unknowns. If the unknowns have not been processed by any digestion method, the blank would be diluted acid of similar characteristics to those of the unknowns. The procedure blank will be the first solution to be run to check the cleanness of the sample preparation procedure. If the blank produces unacceptably high counts, the analyses will not proceed. To avoid wasting samples, use only high purity reagents at all stages of sample preparation. We need to keep the ICP-MS as clean as possible to be able to reach low levels of detection (ppt).
- Standards: The user should provide a set of standards for each project that have concentration range similar to that expected from the unknowns. These standards will be used to generate the calibration curves from which the composition of the unknown samples will be inferred. The user should discuss the number and type of standards most appropriate for the analysis with the lab manager. For best results, the standards should have a similar matrix to the unknowns and be prepared by the same method. If samples and standards are not matrix-matched, the accuracy of results may be compromised. There are two types of standards that are used in solution ICP-MS. One set of standards includes those solutions prepared from single or multi-element standards that are commercially produced. The second set of standards is known as SRM (standard reference material). SRMs are samples with well-defined compositions (working values) from analyses by many different laboratories. A combination of commercially produced standards and natural SRM is encouraged.
- Internal standards: Internal standards (IS) are elements that are added to the blank, standards, and samples in known concentrations. The concentration of a given internal standard should be the same in all the solutions (i.e. blank, standards, and samples).
To select an internal standard keep in mind the following:
- the internal standard should not have isobaric interferences with the analyte(s)
- the samples and standard reference materials should have negligible concentrations of the IS
- when analyzing a group of elements with a wide range of masses, several internal standards should be used with a similarly wide range of masses (e.g. Be, In and Bi)
Internal standards are widely used in ICP-MS analyses to correct for variations in the instrument response as the analysis proceeds (drift) and to calculate the analyte concentrations of the samples (see principles above)